Hydrolysis of nitriles with aqueous acid to give carboxylic acids
Description: Addition of water and acid to a nitrile leads to formation of a carboxylic acid.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-World Examples:
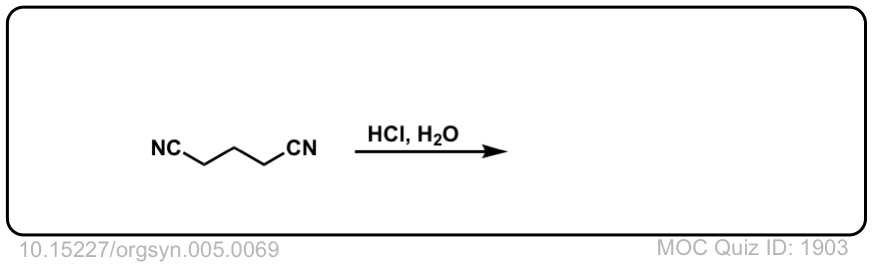
Org. Synth. 1925, 5, 69; Org.
DOI Link: 10.15227/orgsyn.005.0069
 Click to Flip
Click to Flip

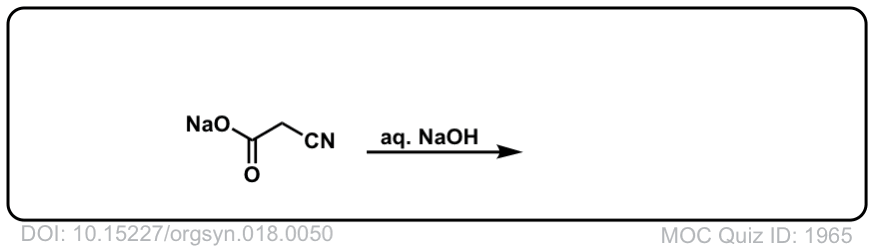
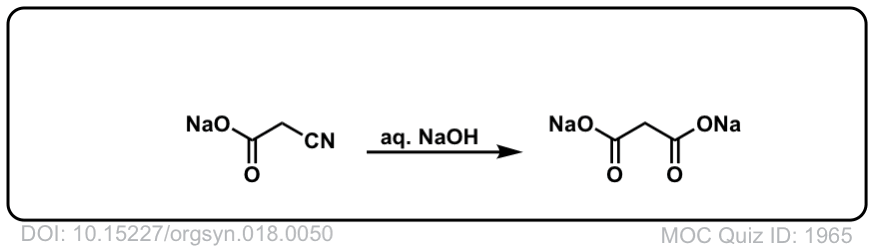
Org. Synth. 1938, 18, 50
DOI Link: 10.15227/orgsyn.018.0050
 Click to Flip
Click to Flip
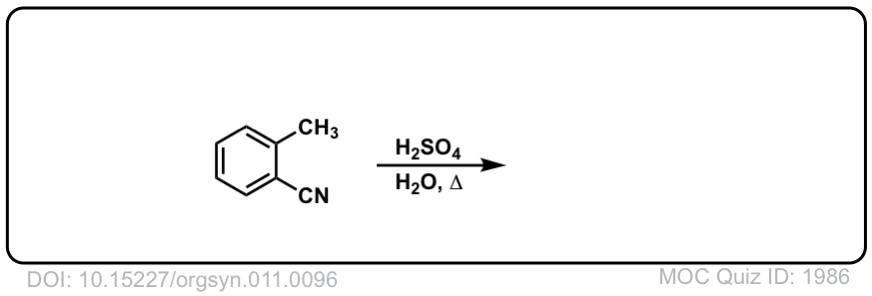
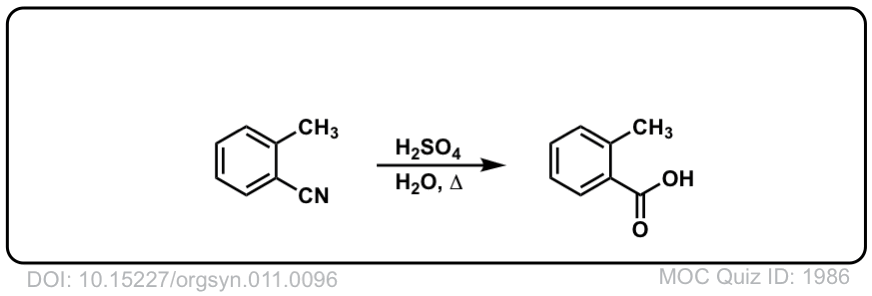
Org. Synth. 1931, 11, 96
DOI Link: 10.15227/orgsyn.011.0096
 Click to Flip
Click to Flip
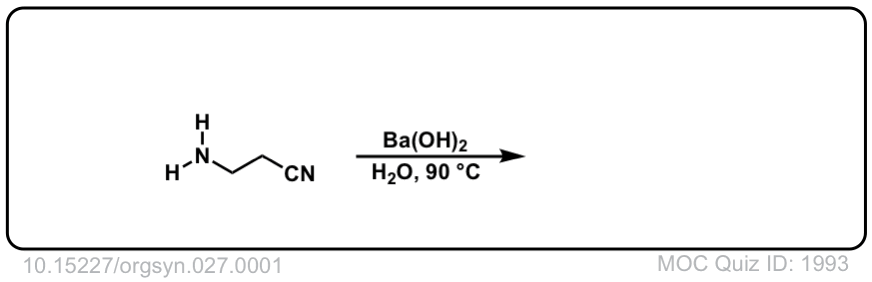
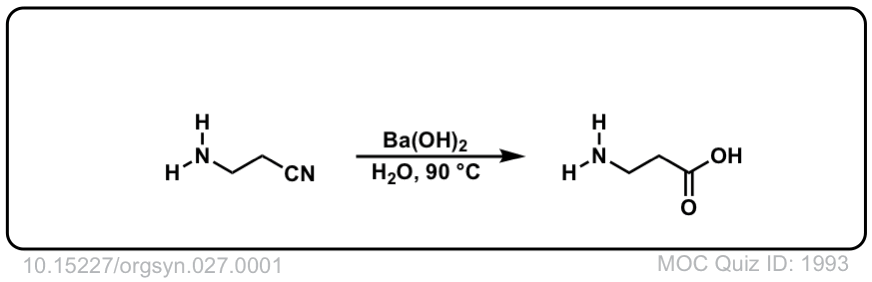
Org. Synth. 1947, 27, 1
DOI Link: 10.15227/orgsyn.027.0001
 Click to Flip
Click to Flip
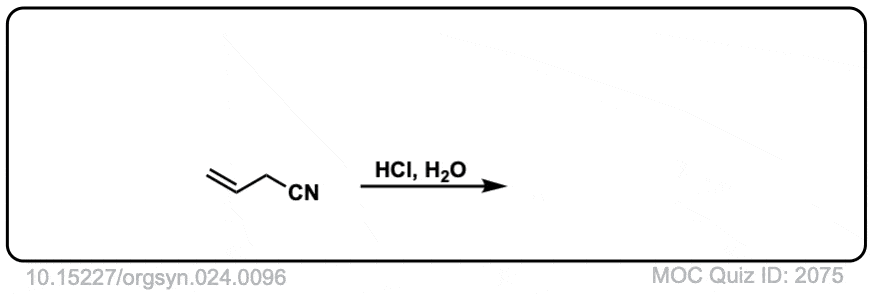
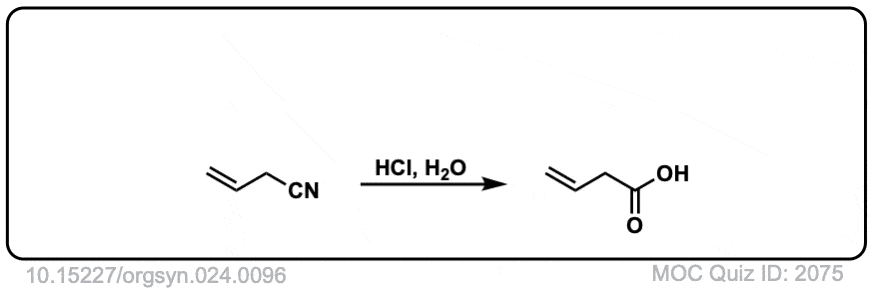
Org. Synth. 1944, 24, 96
DOI Link: 10.15227/orgsyn.024.0096
 Click to Flip
Click to Flip
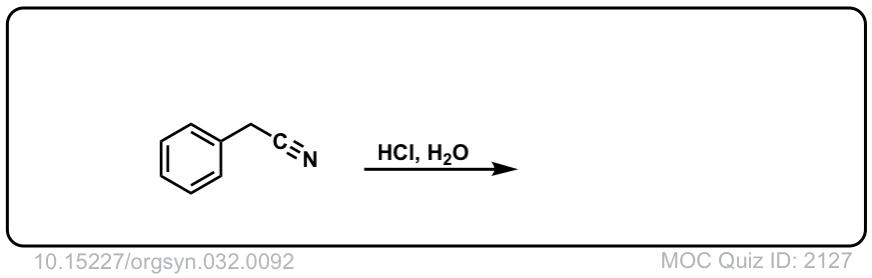
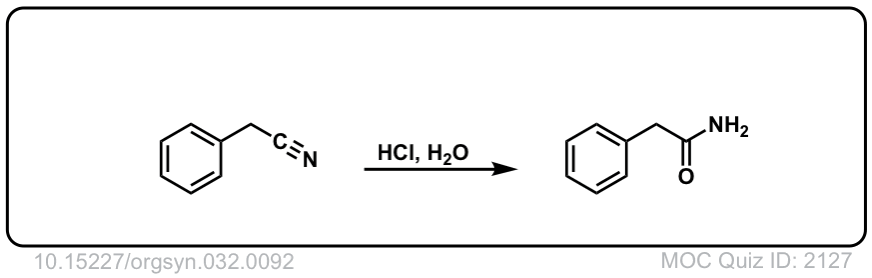
Org. Synth. 1952, 32, 92
DOI Link: 10.15227/orgsyn.032.0092
 Click to Flip
Click to Flip
Thank you James, sorry i meant like in step 3, why is the hydrogen at the bottom positively charged?(the one below step F)
Hi, same idea: in step 2, neutral OH2 goes from owning its own pair of electrons to sharing a pair of electrons with carbon, so it becomes more positive by 1. In step 3 the formal charge on the oxygen is shown as +1, but it will become neutral once it has been deprotonated by the water (acting as a base here). Step 3 will result in the oxygen sharing a pair of electrons with H (in the O-H bond) to owning its pair of electrons again.
Hi also, in step 4, why is the hydrogen at the bottom and the oxygen of the hydronium positively charged? Thank you
Water goes from OH2 (owning both lone pairs) to sharing a pair of electrons with H+ in a new O-H bond, so it loses a negative charge. The result is a positively charged oxygen. 0 – (-1) = +1
Hi, regarding step 2 in the mechanism, why does the nitrogen carry a positive charge? I mean it has gained more electrons shuffle towards the nitrogen right? And also the hydrogen and nitrogen coordinate bonded so the positive wouldn’t be coming from the hydrogen. So where does this positive charge come from?
Nitrogen goes from owning its lone pair to sharing its pair of electrons with H (the new N-H bond). This means it has one less negatively charged electron to itself. It loses a negative charge, going from zero to +1.
Would hydrolysis of nitriles with aqueous acid to give amides first?
Would nitriles become amides first, then carboxylic acids?
Thank you very much.
Yes, that is absolutely correct. It’s possible to stop it at the amide stage.
do you have a base-promoted version of hydrolysis as well?
Base promoted hydrolysis of amides is hard, because eventually the nitrogen has to leave as an anion, and species like H2N- and R2N- are very very strong bases. It can be done, however. https://pubs.acs.org/doi/abs/10.1021/ja00421a046
missing a H on NH4+ -OSO3 in product??
Oops – yes. Sorry, let me fix that. Thanks for spotting
do you have a convenient metod based on articles for this reaction??