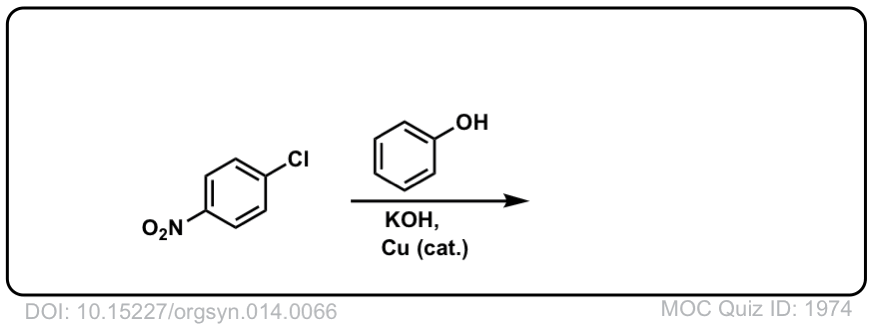
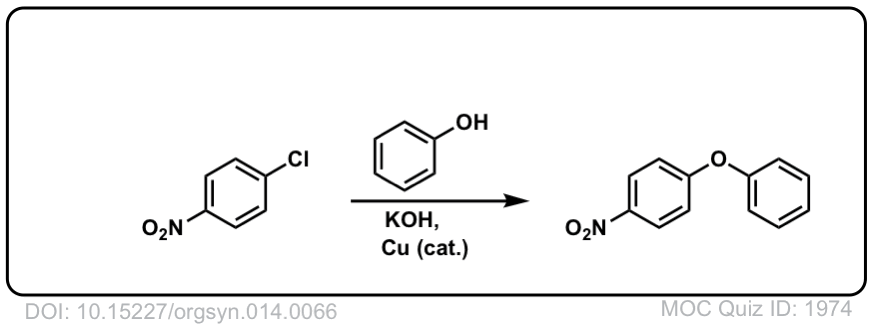
Nucleophilic Aromatic Substitution (SNAr)
Description: Aromatic rings containing electron withdrawing groups and a leaving group can undergo substitution reactions when treated with strong nucleophiles.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1934, 14, 66
DOI Link: 10.15227/orgsyn.014.0066
 Click to Flip
Click to Flip
for question number #4 quiz ID: 1845, why is the nucleophile attached to the meta position? I thought it could only be possible on ortho and para for SNAr
Hi Stephanie – yes, come to think of it you are correct. I’m going to adjust this, since the resulting negative charge after addition of nucleophile wouldn’t be very stabilized.
Thanks!!
For the last diagram showing resonance you said “what about attack at the carbon ‘ortho’ to the nitro group” , but you meant to say meta.
Fixed! thank you