Oxidation of secondary alcohols to ketones using PCC
Description: Treatment of secondary alcohols with pyridinium chlorochromate (PCC) leads to ketones.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-World Examples
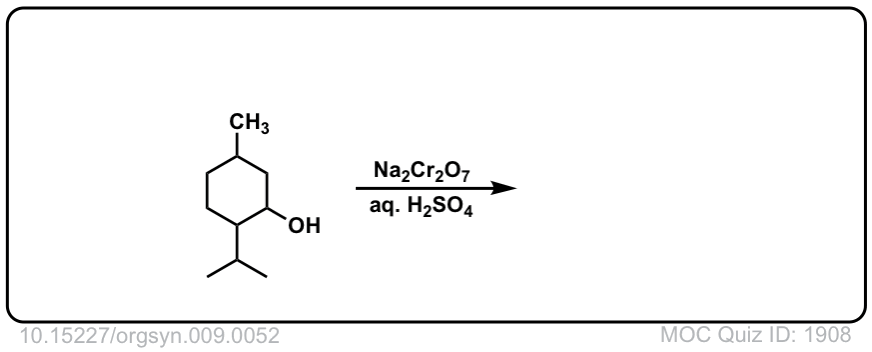
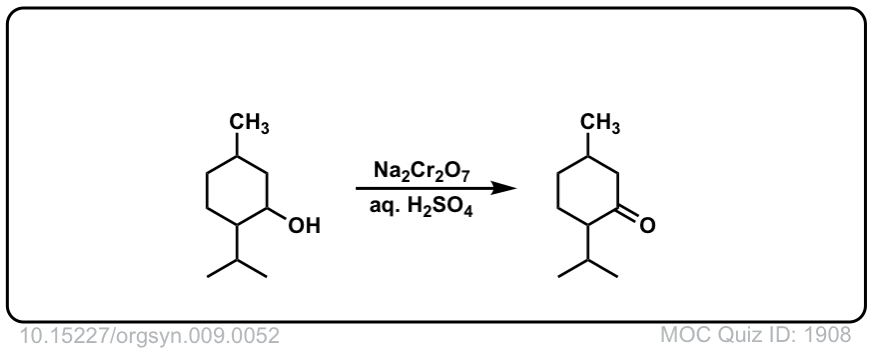
Org. Synth. 1929, 9, 52
DOI Link:
10.15227/orgsyn.009.0052
 Click to Flip
Click to Flip
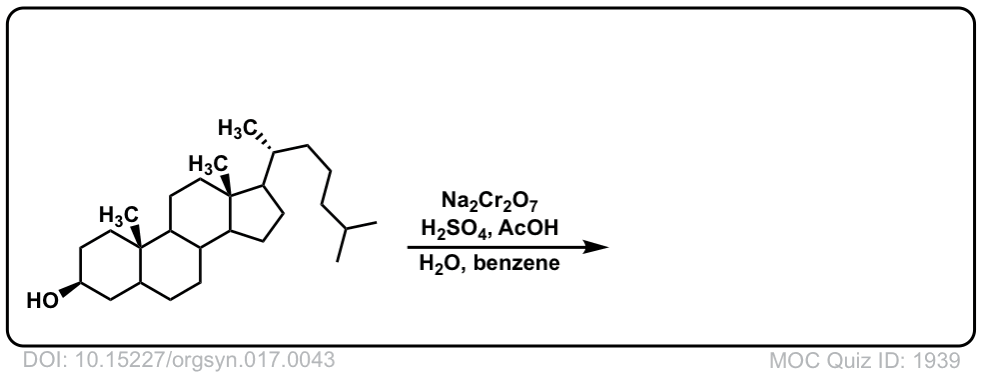
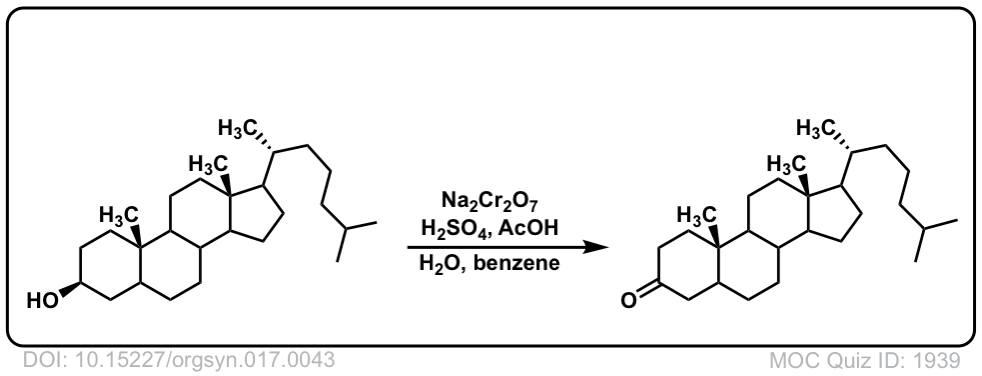
Org. Synth. 1937, 17, 43
DOI Link: 10.15227/orgsyn.017.0043
 Click to Flip
Click to Flip
Org. Synth. 1941, 21, 18
DOI Link: 10.15227/orgsyn.021.0018
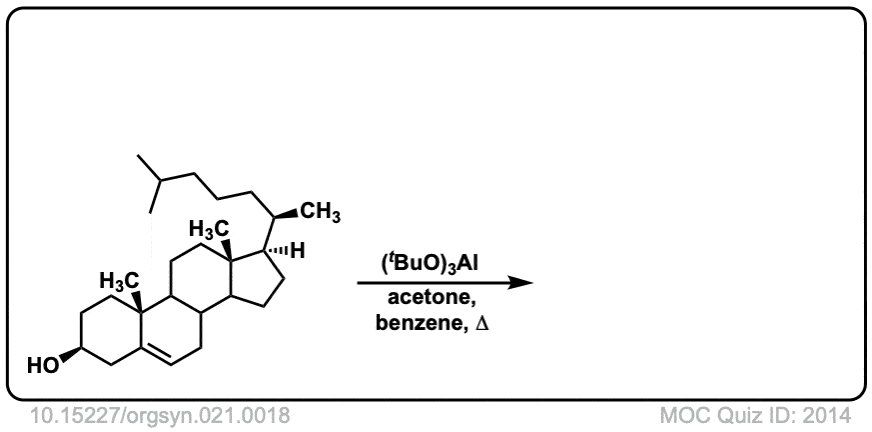 Click to Flip
Click to Flip
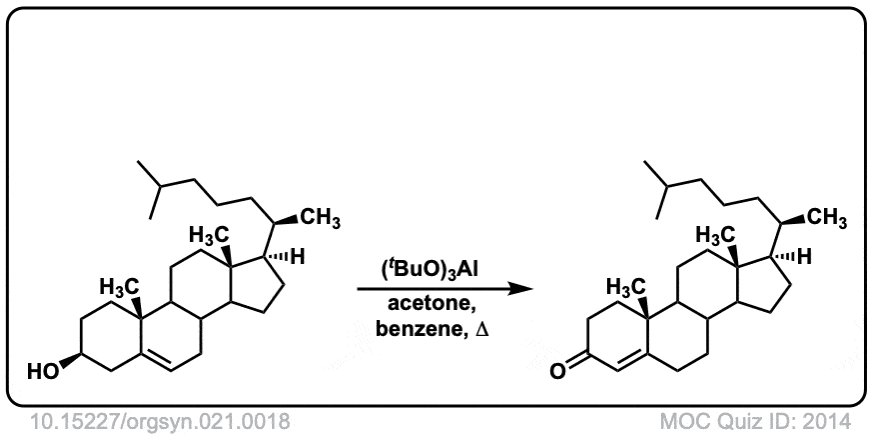
Org. Synth. 1955, 35, 39
DOI Link: 10.15227/orgsyn.035.0039
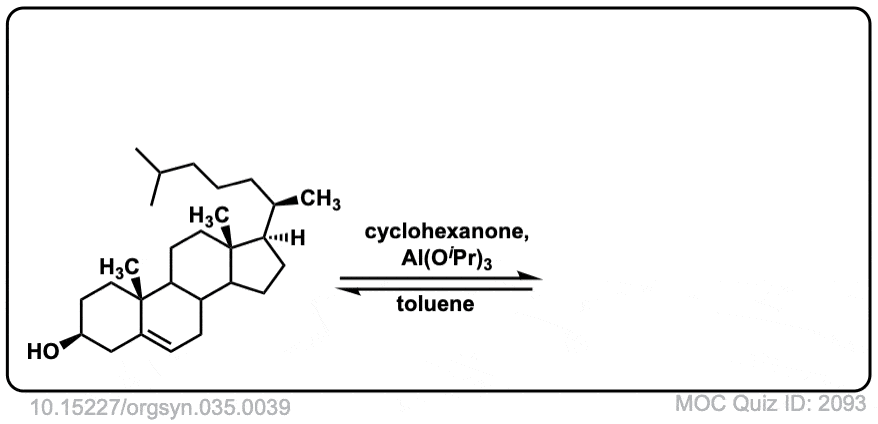 Click to Flip
Click to Flip
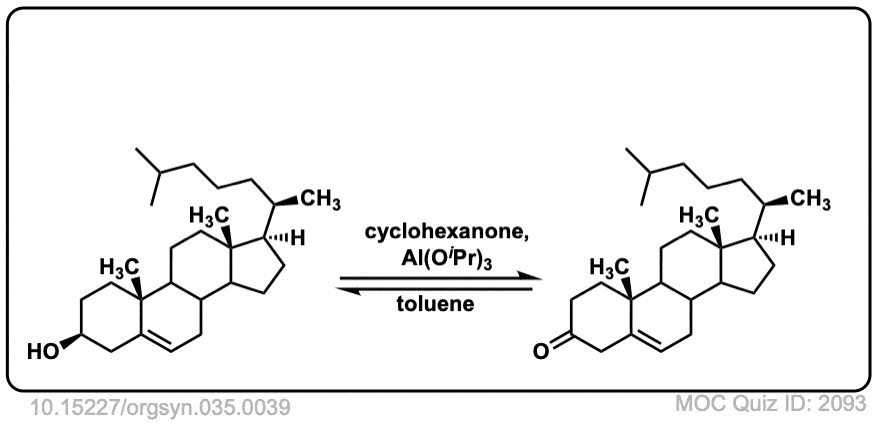
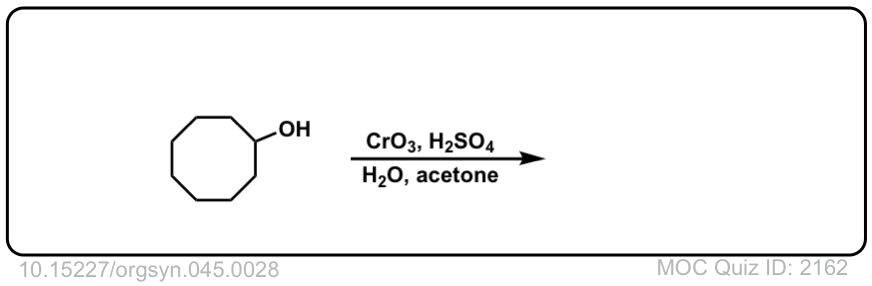
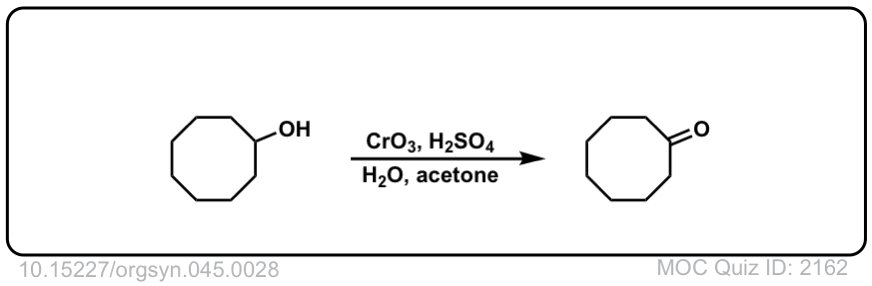
Org. Synth. 1965, 45, 28
DOI Link: 10.15227/orgsyn.045.0028
 Click to Flip
Click to Flip
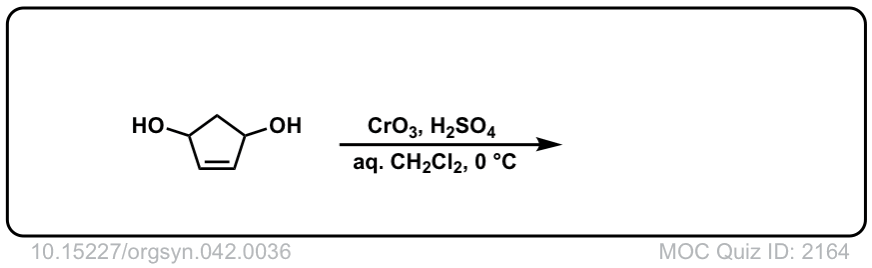
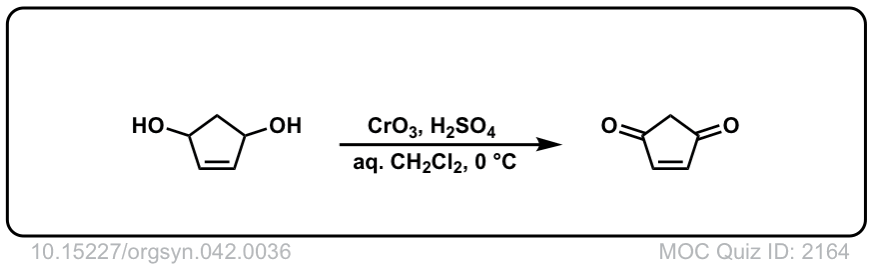
Org. Synth. 1962, 42, 36
DOI Link: 10.15227/orgsyn.042.0036
 Click to Flip
Click to Flip
Hi, is there disadvantages/advantages to using the different reagents (PCC/H2CrO4/Swern Oxidation/KMnO4)?
“On paper” they all carry out equivalent transformations.
In practice…. well, H2CrO4 has toxic chromium (VI) so we try to avoid it, plus it’s strongly acidic which can affect acid-sensitive groups on the molecule. PCC is milder from an acidity standpoint but will tend to form gums/tars at the bottom of your flask, plus it has the toxic chromium issue. The Swern is a nice oxidation to use, especially on large scale, but it does have the disadvantage that you have to run it at -78°C and you generate a lot of foul-smelling dimethyl sulfide (DMS). [a related but well-performing oxidation is done with SO3-pyridine complex, the Parikh-Doering oxidation]. KMnO4 is a wild beast that will also oxidize alkenes, so avoid if at all possible. Dess-Martin periodinane is popular because it’s a weighable white solid that does the job simply and effectively (although it does have a high molecular weight and is probably not best for large-scale applications).
Generally speaking the Parikh-Doering and Dess-Martin Periodinane won’t get you into many problems.
I hope this answers your question! James
How about the selective oxidation of benzylic alcohol via MnO2 ? We use that in class in synthesis problems.
Good suggestion!
If the byproducts are H2CrO3 and pyridinium chloride then bond G should never be formed and the bond J should be a bond formed with O-H
i was convinced with the mechanism of oxidation with chromic acid.. but, i wondered how does pcc work as a selective reagent here?!! now i understand how it does…
Glad you found it helpful Payel.