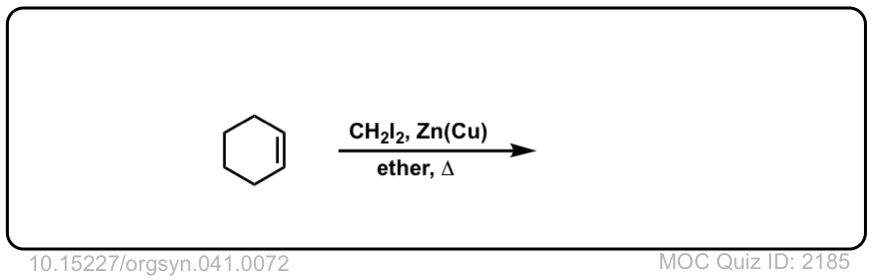
Formation of cyclopropanes from alkenes using methylene carbene (:CH2)
Description: Alkenes treated with methylene carbene (:CH2) will form cyclopropanes. The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-World Example:
Org. Synth. 1961, 41, 71
DOI Link:
DOI: 10.15227/orgsyn.041.0072
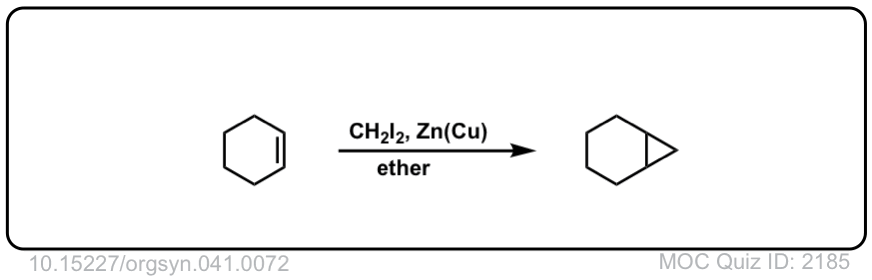
 Click to Flip
Click to Flip
Hello, for the 3rd to last flashcard on this page, from what I observe, the reagents CHCl3 and KOt-Bu are used to stimulate Dihalocyclopropanation involving an alkene. If that is true, why doesn’t the epoxide ring in this case contain 2 chlorines attached to a carbon in the final product? What makes the 2nd to last flashcard have chlorine atoms attached to the carbon on the epoxide ring instead? Thanks.
Woops! That’s a typo. Should have the chloros attached. Will fix.
why do you have bromine addition images here?
I think he linked it to the wrong photo on the site…just ignore it until he fixes it.
Why do carbenes not react with alkynes? Has it got to do with the angle strain in the product which makes it unstable?
Also, how do ZnCu react with CH2I2? Is ZnCu a catalyst? Where can I find the mechanism?
Carbenes can react with alkynes to give cyclopropenes, but it’s a slower reaction due to the fact that the products (cyclopropenes) have more ring strain. Generally cyclopropanation of alkynes is not covered in these introductory courses, so it isn’t mentioned here.
wow it was a very nice information!!!!
i just gone through all the reactions of alkenes it is very helpfull for me!!!
thank you for uploading them in website!!!!
Glad you find it useful.